New Version Launch
We are excited to announce that the launch event for our latest an updated version of eIRB, eIRB 2.0 was a huge success! Thank you to everyone who attended and helped make this event memorable. If you missed it, don’t worry – the good news is that eIRB2.0 is officially launched and will be implemented soon!
What You Can Expect:
- Powerful New Features: eIRB 2.0 is packed with innovative features designed to streamline your study. Whether you're looking to increase productivity, improve efficacy, or enhance your workflow, our software has it covered.
- Seamless Implementation: We’re working hard to make sure that eIRB 2.0 is implemented smoothly and is available to all users in the coming weeks. Our team is dedicated to making sure the rollout is as seamless as possible.
- Support and Resources: To ensure a smooth transition, we’ll be providing plenty of resources, including tutorials, user guides, and a dedicated support team ready to assist you every step of the way.
What’s Next?:
- Implementation Timeline: The official rollout of eIRB2.0 will begin on 1st April 2025. Be ready to experience its full potential very soon!
- Stay Updated: We’ll be sharing updates and important details leading up to the implementation date. Be sure to keep an eye on your inbox and our website for more information.
- Have Questions: If you have any questions about the software or the upcoming implementation, our team is always here to help.
Why We’re Excited:
- eIRB 2.0 is designed to improve your experience with QCR. With its powerful features and user-friendly interface, it’s set to become an essential tool for our clients.
- We’re confident that once eIRB 2.0 is fully implemented, it will make a significant impact on your study/trial related workflow and help you achieve your goals more effectively.
Stay Connected: Follow us on social media to keep up with the latest updates and announcements regarding the implementation process.
Streamlining Ethics Committee Submissions
e-IRB Digital Applications: Streamlining Ethics Committee Submissions
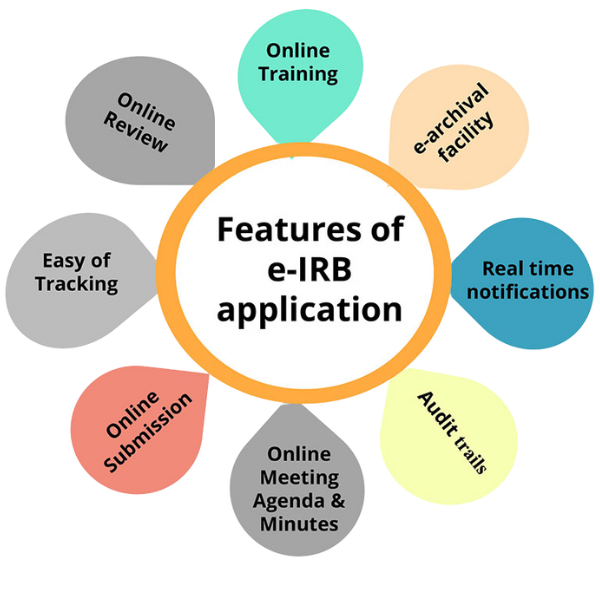
Online Training
This feature provides users with accessible training modules directly through the e-IRB platform. It aims to empower users by offering educational resources, tutorials, and guidelines specific to the IRB submission process.
e-Archival Facility
A secure, state-of-the-art facility document storage facility for safekeeping of all documents in a digital format, reducing the risk of loss or damage. Ensuring easy retrieval of documents, aiding in efficient record-keeping and reference.
Real-time Notifications
The platform sends timely notifications to all stakeholders involved in the IRB process. Keeps users informed with email and SMS alerts about application status, meetings, and updates reducing delays and improving communication flow.
Audit Trials
Audit trails are an essential feature for ensuring transparency and traceability within the e-IRB process. They provide a detailed record of all actions taken within the system, including who performed them and when fulfilling compliance purposes.
Online Meeting Agenda & MoM
e-IRB streamlines the scheduling and documentation of meetings related to the IRB process. Online access to meeting agendas and MoM ensures that all participants are on the same page and can prepare adequately for discussions.
Online Submission
The platform enables a streamlined and simplified process for submitting documents to the IRB. This online submission process reduces paperwork, saves time, and increases efficiency, especially.
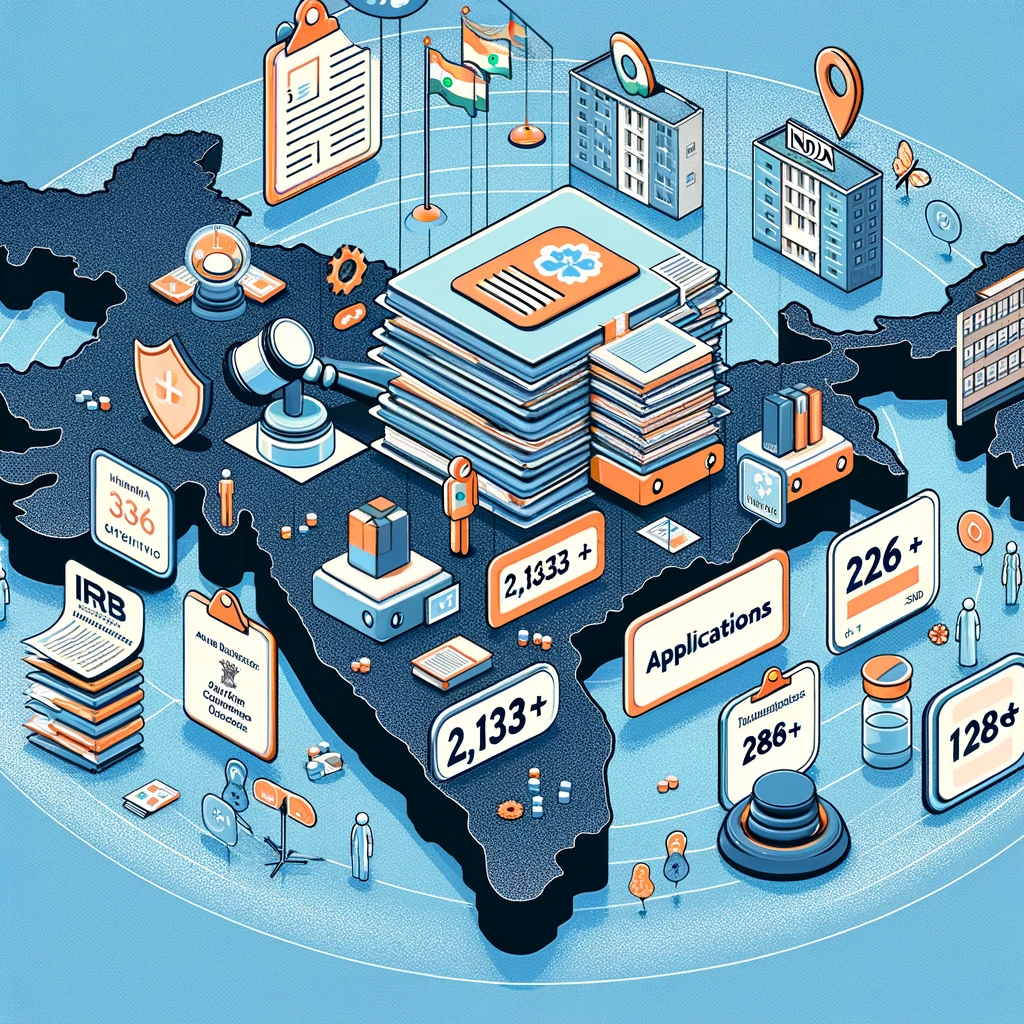
Expansive Reach Across India
Significant impact in managing research applications across multiple Ethics Committees and hospitals. The platform's success in handling a large number of applications across institutions highlights its effectiveness in improving the efficiency, transparency, and standardization of the IRB review process in clinical research.
0+
5287 applications - The eIRB platform has processed a total of 2,133 applications.
0+
151 Ethics Committees - The platform has been utilized by 134 different Ethics Committees.
0+
303 hospitals - These applications span across 286 hospitals illustrates the platform's extensive reach.

Comprehensive Benefits for All Stakeholders
Empowering Each Stakeholder with Streamlined Processes, Enhanced Compliance, and Advanced Technology Solutions
Full-Spectrum Benefits for All Involved
Experience the synergy of collaborative success—where every stakeholder enjoys tailored advantages, fostering growth and innovation across the entire ecosystem.
Streamlined Tracking & Submission
Navigate the complexity of clinical trials with ease. Our eIRB system simplifies tracking and submission, making your research journey smooth and efficient.
Accelerated Review Processes
Speed up your path to discovery. Our eIRB platform expedites the review process, ensuring your research moves forward swiftly and smoothly.
Guaranteed Regulatory Compliance
Stay ahead with confidence. Our eIRB system ensures your research adheres to all regulatory standards, keeping you compliant and protected.
Enhanced Documentation Accessibility
Access your research documents anytime, anywhere. Our eIRB platform enhances documentation accessibility, supporting your research needs on the go.
Unmatched Data Security
Protect sensitive information with our robust eIRB system. Experience peace of mind knowing your data is secured against unauthorized access.
Comprehensive Archival Compliance
Preserve your research legacy. Our eIRB platform provides long-term archival solutions, ensuring compliance and safeguarding your work for future generations.
Comprehensive Services of e-IRB
e-IRB offers a wide array of services to streamline and enhance the efficacy of IEC operations
E-registration and Login ID Creation
Simplifies the process of user registration and login ID creation for IECs, sponsors, CROs, and pharmaceutical companies, ensuring secure and personalized access to the system.
Detailed IEC Information
Provides comprehensive information on registered Independent Ethics Committees (IECs) by city, state, or country, offering users easy access to crucial data for informed decision-making.
Access Anywhere
Offers a flexible login facility with unique IDs and passwords, enabling users to access the system from any location at any time, enhancing convenience and operational flexibility.
IEC Member Activities
Facilitates active participation of IEC members in their respective roles, allowing them to efficiently perform tasks and responsibilities assigned by the IEC through the platform.
Clinical Trial Application Management
Streamlines the process of submitting and managing clinical trial applications for IEC review and approval. This feature ensures an efficient and organized approach to application handling and compliance.
User Account Management
Incorporates automated features for user account creation, modification, deactivation, and managing access rights, ensuring efficient and secure user management.
Record Generation and Management
Enables the generation and management of various essential IEC records, including Meeting Agendas, Minutes of Meetings (MoM), and decision-making documents, ensuring organized and compliant record-keeping.Value
Reporting and Updates
Provides a robust system for submitting and reporting study progress, updates, and schedules, as well as managing serious adverse events (SAEs) or adverse events (AEs), and protocol deviations, ensuring up-to-date project tracking.
Communication and Alerts
Implements an effective communication system, sending timely email and SMS notifications for application uploads, updates, meeting schedules, and other critical alerts, ensuring all stakeholders are well-informed.
Reach Out for Support and Information
Have Questions or Need Assistance? Our Dedicated Team is Ready to Provide Expert Support and Guidance. Contact Us Today for Personalized Help and Detailed Information on Our Services.